Gary Eitzen

Gary Eitzen
Ph.D., University of Alberta
Professor
Office: 5-23A Medical Sciences Building
Laboratory: 5-23 Medical Sciences Building
Telephone: 780-492-6062
RESEARCH INTERESTS
Project I: Rac signal transduction and control of inflammatory cells exocytosis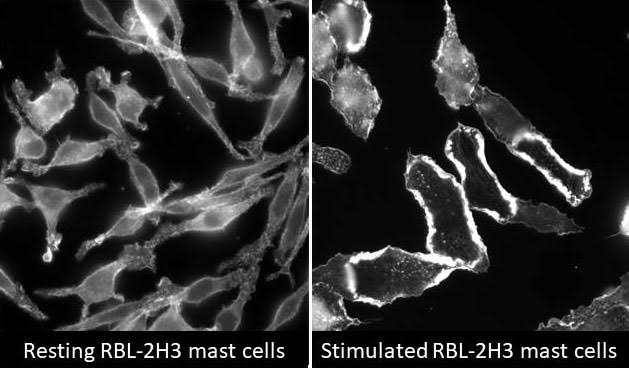
Innate inflammatory cells such as neutrophils, eosinophils, basophils and mast cells are the primary hematopoietic effector cells involved in early immune responses. These cells are also known as granulocytes and have numerous cytoplasmic granules that package potent pro-inflammatory mediators. During infection or an allergic reaction, they contribute to inflammation and tissue damage by degranulating in response to activation, resulting in release of mediators including chemoattractants, cytokines, chemokines and cytotoxic enzymes contained in intracellular granules. Degranulation occurs by regulated exocytosis (granule-plasma membrane fusion) of granules in response to cell activation. Therefore, an effective anti-inflammatory therapy or to modify an allergic response would be one that is directed at downregulation of inflammatory cell exocytosis. Several key signaling molecules have been implicated in the regulation of exocytosis from granulocytes. Rho proteins and their associated signaling pathways represent ideal targets for allergic response therapy; in particular, since many Rho signaling molecules are selectively expressed in immune cells. Our hypothesis is that inhibition of key regulatory pathways leading to inflammatory cell exocytosis may provide ideal therapy for modulation of an allergic response.
Project II: Control of cytokine upregulation in lung epithelial cells
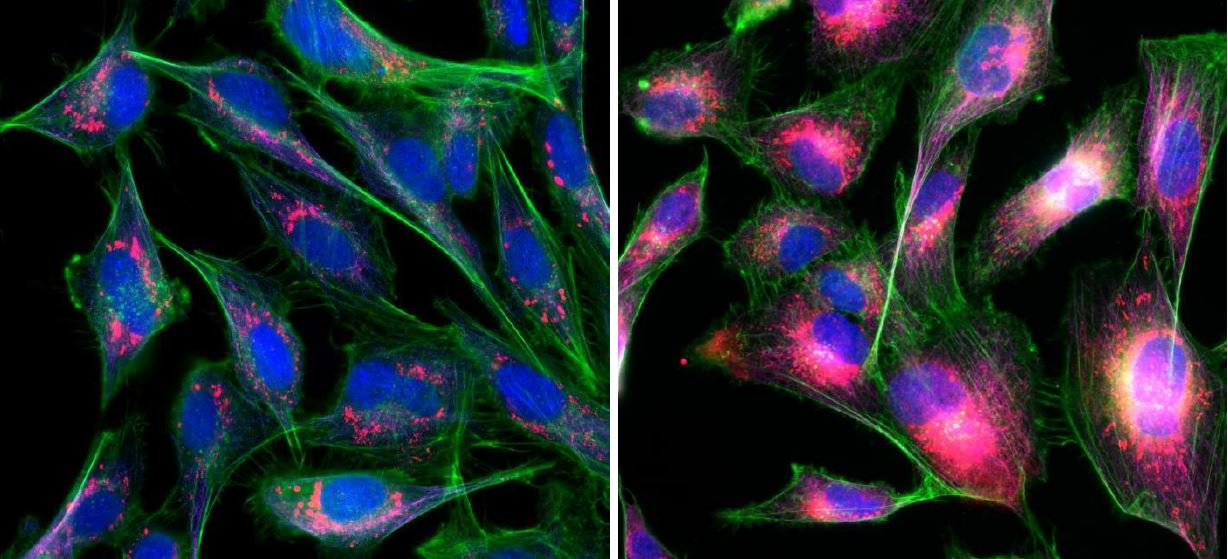
BEAS-2B cells resting (left) or TNFα-stimulated for 4 hr (right)
IL-8 (red), F-actin (greeen), DNA (blue)
BEAS-2B cells are an immortalized human bronchial epithelial cell line that has been widely used to model respiratory diseases. We are examining the activation of inflammation in BEAS-2B cells after treatment with different allergens and stimulants such as cockroach extract (allergen), polyinosinic-polycytidylic acid (viral mimetic), and TNF-α (general inflammation). We assess the state of inflammation by quantifying the production and release of pro-inflammatory cytokines. We have found that Rho proteins act as signal transduction mediators in the pro-inflammatory pathways in lung epithelial cells. Further studies are aimed at determining whether Rho proteins contribute to dysfunctional regulation of pro-inflammatory cytokine production in a variety of lung diseases.
Selected Publications
Mast cell granule motility and exocytosis is driven by dynamic microtubule formation and kinesin-1 motor function.Ibanga J, Zhang EL, Eitzen G, Guo Y. PLoS One. 2022 Mar 22;17(3):e0265122.
FGD5 regulates endothelial cell PI3 kinase-β to promote neo-angiogenesis.
Azad AK, Farhan MA, Murray CR, Suzuki K, Eitzen G, Touret N, Moore RB, Murray AG. FASEB J. 2022 Jan;36(1):e22080.
Laboratory Members
Graduate Students and Trainees
Rowayna Shouib