Exercise Oncology Research Laboratory

The Exercise Oncology Research Laboratory is focused on generating new knowledge on how physical activity can help cancer patients prepare for treatments (prehabilitation), cope with treatments, recover after treatments (rehabilitation), and improve long term quality of life and survival.
The Exercise Oncology Research Laboratory, in particular, evaluates patients' exercise behaviour and physical fitness before and after treatment.
Our research seeks to advance the scientific understanding of the interrelationships among the behavioural, biological and psychosocial aspects of physical activity and cancer.
Who We Are
The laboratory is overseen by Dr. Kerry S. Courneya, Director of the Exercise Oncology Research Laboratory, Canada Research Chair in Physical Activity and Cancer, and a Professor in the Faculty of Kinesiology, Sport, and Recreation.
The Exercise Oncology Research Laboratory works closely with the Cross Cancer Institute in Edmonton. The Institute's experts assist us in our work at the Centre.
Graduate Student Opportunities
Dr. Courneya typically supervises 4-8 graduate students and post-doctoral fellows in the Exercise Oncology Research Laboratory (EORL) and opportunities are available regularly.
- Most students in the EORL hold external scholarships or receive full research assistantships (RAs) as part of their training
- Students work 12 hours per week in the EORL
Prior to applying for either graduate studies or a post-doctoral fellowship, those interested should contact Dr. Courneya to determine if their research interests fit with those of the EORL, and also what opportunities are available for supervision.
Contact Dr. Kerry Courneya
Email: kerry.courneya@ualberta.ca
Phone: 780-492-1031
About the Laboratory

The EORL is located in the Edmonton Clinic Health Academy (ECHA) and is fully equipped with the latest exercise equipment.
The Centre is equipped with:
- Life Fitness treadmills,
- Life Fitness recumbent and upright cycle ergometers,
- APEX selectorized resistance machines.
- Other equipment also includes hand weights, exercise balls, and a stretching area.
The EORL operates primarily by appointment.
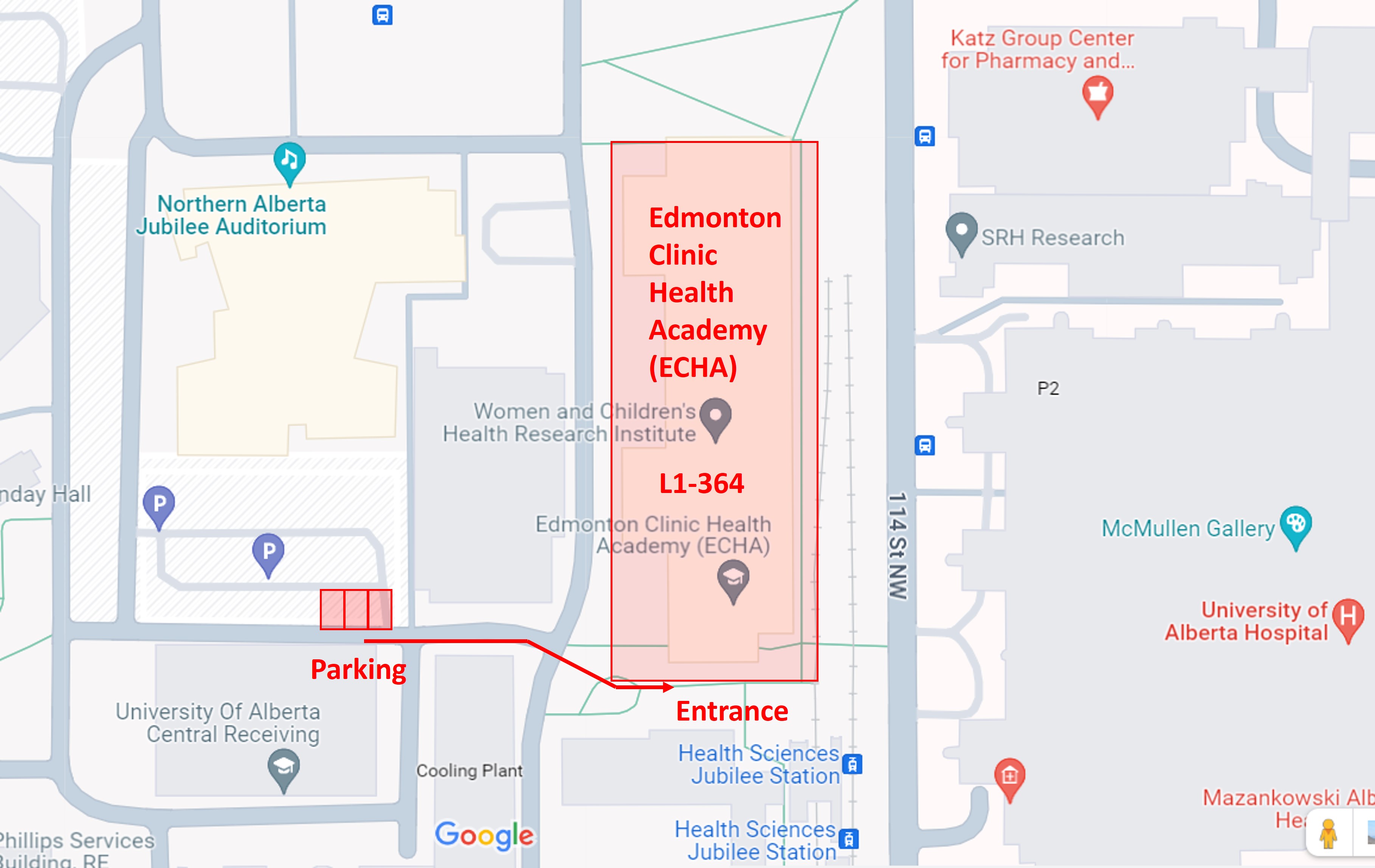
Parking
Study participant parking cost are covered by the EORL. Participants are not charged for parking. Our parking spots are on the west side of the building, in the Jubilee Surface parking lot (west side of Jubilee Car Park).
If parking stalls are full, please purchase a ticket at the ticket booth and we will reimburse you.
- Cost is $4.50 per hour at Jubilee Surface Parking Lot.
How to get to the Exercise Oncology Research Laboratory

The Exercise Oncology Research Laboratory is located at the University of Alberta, west of the Health Sciences LRT Station, in the Edmonton Clinic Health Academy Pavilion (11405-87 Ave NW).
The EORL is in rooms L1-364 and L1-370. From the Jubilee Surface lot, the best way to get access is through the south doors of the ECHA building.
According to the study in which the participant is in, different components of physical fitness will be assessed. These may include:
- Dual energy x-ray absorptiometry (DXA): It is a non-invasive technique to measure bone density (AP spine and femur), lean tissue mass, and total and regional body fat (i.e., abdominal body fat). This body composition measure requires the participant to wear a hospital gown and lie quietly on the apparatus for approximately five minutes while an external scan of the entire body is performed.
- Anthropometrics measures: Body weight, height, hips and waist circumferences.
- Physiological test: To perform those graded exercise tests, our Exercise Testing Room are equipped with an:
- Ergoline Electronically Braked Bike and Woodway Treadmill for the aerobic exercise tests
- Metabolic measurements are determined with the Parvo Medics TrueOne® 2400 Metabolic Measurement System
- Physical functioning: lower and upper body strength; lower and upper body flexibility, agility, and aerobic endurance.
We conduct multidisciplinary, biopsychosocial research in three broad areas:
- The effects of exercise on cancer control outcomes such as prevention, coping, rehabilitation, health promotion, palliation, and survival,
- the determinants of exercise for cancer control, and
- the effectiveness of interventions designed to promote exercise for cancer control.
The Exercise Oncology Research Laboratory is involved in many large scale case-control and cohort studies as well as single-site and multi-centre randomized controlled trials, with a variety of cancer sites being represented, including colorectal, prostate, testicular, hematologic, gynecologic, and breast cancer.
A protocol of precautions has been implemented for fitness testing and the exercise intervention including protective equipment during testing and masks for supervisors if required, and a limited number of study participants in our facility at one time. Moreover, equipment and facilities are disinfected regularly before and after each testing/exercise intervention.
Ongoing Research Studies
Here is a sample of some of the studies that are in progress.
CIHR Team in Physical Activity + Breast Cancer Survivorship: The Alberta Moving Beyond Breast Cancer (AMBER) Cohort Study
Research Description
The objective of our research team is to study physical activity (PA) and health-related fitness (HRF) to improve breast cancer survivorship from the time of diagnosis and for the balance of life. We propose to achieve this objective by establishing a large cohort of breast cancer survivors with a comprehensive assessment of subjective and objective measures of physical activity and health-related fitness (e.g., physical fitness, physical functioning, body composition). This cohort will allow us to address important questions related to physical activity and health-related fitness including (a) examining associations with important outcomes such as disease outcomes, symptoms, late effects, psychosocial outcomes, and quality of life, (b) examining determinants such as medical, social cognitive, and environmental, and (c) examining mechanisms and moderators of observed associations. These data will help inform strategies to promote physical activity and health-related fitness to improve breast cancer survivorship.
Our study will include:
- a comprehensive self-report measure of PA developed by our team that measures the type, frequency, intensity, and duration of PA at work, at home, and for recreation and transportation
- a self-report assessment of sedentary behavior (e.g., television viewing, sitting time) that has emerged as an important independent predictor of disease outcomes in other populations including the risk of developing ovarian cancer
- state-of-the-science objective measures of PA and sedentary behavior (i.e., accelerometers)
- a comprehensive assessment of HRF including standardized and validated measures of cardiorespiratory fitness, musculoskeletal fitness, and body composition
- a full assessment of biomarkers purported to mediate possible associations between PA, HRF, and breast cancer outcomes
- a comprehensive assessment of PROs including quality of life, fatigue, and cognitive function using standardized and validated measures
- a complete assessment of potential determinants of PA and HRF based on a social ecological model that includes social cognitive and environmental correlates.
The cohort study will provide the most comprehensive inquiry into the role of PA and HRF in breast cancer survivorship to date. The data generated will allow us to answer key questions related to PA and HRF in breast cancer survivors including:
- the independent and interactive associations of PA and HRF with important health outcomes in breast cancer survivors including disease outcomes (e.g., recurrence, breast cancer-specific mortality, overall survival), treatment completion rates, symptoms and side effects (e.g., pain, lymphedema, fatigue, cognitive dysfunction), and PROS (e.g., QoL, anxiety, depression, self-esteem, happiness)
- the determinants of PA and HRF including demographic, medical, social cognitive, and environmental variables
- the mediators of any observed associations between PA, HRF, and health outcomes including biological, functional, and psychosocial outcomes
- the moderators of any observed associations including demographic, medical, and biological factors.
The AMBER cohort will serve as the basis for five initial research projects proposed within our CIHR Team Grant. The overall aim is to establish a cohort of breast cancer survivors in whom the role of PA, HRF in breast cancer outcomes can be examined. Specific objectives have been established for the five initial projects that will use this cohort. This large cohort will be built in Alberta over approximately a 4 year period with assessments at multiple time points using subjective and objective measures of PA, HRF, and health.
Overview of Study Design and Methods
A prospective cohort study is proposed that will recruit 1500 survivors of incident, histologically-confirmed, primary breast cancer from Edmonton (i.e., Cross Cancer Institute) and Calgary (i.e., Tom Baker Cancer Centre) who will be followed for a minimum of five years postbaseline assessment. A rapid case ascertainment method using the Alberta Cancer Registry (ACR) will be used to identify cases prior to surgery and to enroll them in this study. Assessments will be made at baseline (within 2 months of surgery and generally prior to the initiation of adjuvant therapy), and at 1 and 3 years follow-up of their PA, HRF, PROs, determinants of PA, and lymphedema through a combination of objective and self-reported measurements. PA, PROs, and determinants of PA will be followed up at 5 years. Blood samples will also be taken at baseline, 1 and 3 years follow-up. The cohort members will be followed up by regular vital status linkages and through chart, and abstractions to identify progressions, recurrences, and new primaries that occur in this cohort. Five projects are initially proposed that will use this cohort to examine separate scientific questions regarding the associations between PA, HRF, and breast cancer outcomes.
Additional Information
Email: amberstudy@ualberta.ca
Exercise is beneficial for breast cancer survivors in alleviating treatment side effects, improving quality of life, and possibly survival. Exercise guidelines for cancer survivors recommend 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic exercise per week, and 2 days of resistance training. However, the number of breast cancer survivors meeting those guidelines declines after treatment because of symptoms such as fatigue, depression, muscle loss, and treatment side effects. Mobile applications (apps) are promising tools with the potential to overcome barriers to exercise such as cost, accessibility, and travel. Since 2020, the Cancer Exercise app has been freely available to cancer survivors but has not been tested to see if it is effective. This study will examine whether a mobile app increases exercise levels in breast cancer survivors after treatments, reduces fatigue, and improves physical functions. This experimental study has 200 participants placed into 1 of 2 groups. One group will be asked to use the Cancer Exercise app over 12 weeks and the other group will receive the exercise guidelines. Exercise will be measured with an objective device called an accelerometer and by self-report questionnaires before and after the intervention and 6 months later. Self-report questionnaires will measure fatigue and quality of life. This research will determine whether a simple mobile app helps increase exercise in breast cancer survivors. Mobile apps to increase exercise may be provided to all breast cancer survivors after treatments to help them recover and possibly improve survival.
Background
Observational studies indicate that physical activity (PA) is strongly associated with improved disease outcomes in colon cancer survivors, but a randomized controlled trial is needed to determine whether the association is causal and whether new policies to promote exercise are justified.
Purpose
The CO.21 Colon Health and Life-Long Exercise Change (CHALLENGE) trial undertaken by the National Cancer Institute of Canada Clinical Trials Group (NCIC CTG) is designed to determine the effects of a structured PA intervention on outcomes for survivors of high-risk stage II or III colon cancer who have completed adjuvant therapy within the previous 2-6 months.
Methods
Trial participants (n = 962) will be stratified by centre, disease stage, body mass index, and performance status, and will be randomly assigned to a structured PA intervention or to general health education materials. The PA intervention will consist of a behavioural support program and supervised PA sessions delivered over a 3-year period, beginning with regular face-to-face sessions and tapering to less frequent face-to-face or telephone sessions. The primary endpoint is disease-free survival. Important secondary endpoints include multiple patient-reported outcomes, objective physical functioning, biologic correlative markers, and an economic analysis.
Summary
Cancer survivors and cancer care professionals are interested in the potential role of PA to improve multiple disease-related outcomes, but a randomized controlled trial is needed to provide compelling evidence to justify changes in health care policies and practice.
Exercise is known to be safe and result in improved physical function and quality of life for most individuals with cancer. However, we know very little about whether exercise can increase overall survival and reduce disease progression, skeletal-related events and pain in patients with metastatic castrate-resistant prostate cancer.
There are 2 arms which patients can be randomized onto on the INTERVAL Trial. Participants on both arms will receive usual care from their doctor, newsletters, questionnaires and periodic fitness testing. Those on the intervention arm will also perform 2 years of supervised exercise sessions made up of high intensity aerobic training and resistance training. The trial is worldwide multi-site trial looking to have 866 people join the trial - 45 of those will be from Edmonton.
The INTERVAL Trial will help us determine if high intensity aerobic and resistance training plus psychosocial support increases overall survival compared to psychosocial support alone in prostate cancer patients. The INTERVAL Trial is proposing the use of exercise as medicine, concomitant with other therapies, to improve quality of life, improve drug efficacy, change tumor biology, and ultimately increase and prolong survival in metastatic castrate-resistant prostate cancer patients.
Bladder cancer is the fifth most common cancer in Canada and has the eighth highest cancer mortality rate. The treatment for the most frequent type of bladder cancer is surgically removing the tumour followed by six weeks of medication placed within the bladder (intravesical therapy).
There are physical and psychosocial challenges from bladder cancer and its treatment that may affect how patients feel and function, and consequently their quality of life. Moreover, bladder cancer patients are at a high risk of their bladder cancer recurrence and progression. Exercise is a low-cost intervention that may help patients feel better, manage side effects related to treatment, and improve quality of life. To date, however, no study has examined if it is safe or even possible for bladder cancer patients to exercise during intravesical therapy.
The Bladder cancer and exeRcise trAining during intraVesical thErapy (BRAVE) Trial will be the first study to test the safety and feasibility of exercise in bladder cancer patients during this drug therapy. We will ask some patients to do a supervised exercise program during their drug treatment while other patients will be asked not to exercise. We will compare the 2 groups on how they fare with their bladder cancer treatment. The BRAVE study will provide information on whether exercise may help patients feel better, function better, and possibly even lower their chances of the disease coming back or getting worse.
Despite improvements in treatments, head and neck cancer survivors (HNCS) still endure numerous acute and chronic side effects. Strength training has been shown to manage some of these side effects but most interventions have involved light-to-moderate resistance training programs. Heavy lifting strength training (HLST) may produce better outcomes but it is unknown if such a weight training program is feasible and safe for HNCS.
The primary aim of this proposed study is to examine the feasibility and safety of a HLST program in HNCS at least 1 year post-surgical neck dissection.
This single arm feasibility study will recruit 15-20 HNCS to complete the HLST program 2 times per week. Eligibility criteria are: 1) previously diagnosed with any subtype and stage of HNC; 2) at least 1 year post-neck dissection for HNC and showing full shoulder range of motion or recovery of the SAN as deemed adequate by neurological evaluation; 3) adults ages 18 and up; 4) no unmanaged medical conditions, alcohol, or drug abuse; 5) approved for a HLST program by the treating surgeon and a certified exercise physiologist; 6) ability to understand and communicate in English.
The primary feasibility outcomes will include the eligibility rate (with reasons for ineligibility), recruitment rate (with reasons for refusal), 1RM testing rate (with reasons for not completing the test), program adherence (including attendance, dose modifications, and progression), and follow-up assessment rate (with reasons for drop out). The primary efficacy outcome will be strength gains from baseline. Secondary efficacy outcomes will include physical functioning, quality of life, post traumatic growth, fear of cancer recurrence, pain, body composition, anxiety, fatigue, stress, shoulder mobility, self-esteem, sleep, and motivation to engage in a heavy lifting strength training program.
Weight training is an effective intervention in HNCS but the optimal weight training prescription is unknown. Heavy weight training has never before been explored in this cancer population; making the LIFTING trial the first of its kind. The development of this research idea stems from my 3 HNC experiences, and benefits from the sport of powerlifting. If HLST is deemed safe and feasible in HNCS, it can be compared to light-to-moderate load weight training to determine if it is a better prescription for improving outcomes that are important to HNCS. Additionally, future research may consider exploring this training style in other cancer types. For more information, contact Stephanie Ntoukas at ntoukas@ualberta.ca.
A prospective phase II study of Inguinal Node Sparing Radiotherapy for patients with Early stage Anal Cancer (INSPIRE).
The INSPIRE trial is led by Kurian Joseph, Associate Professor/Radiation Oncologist in the Department of Oncology, at the University of Alberta and Cross Cancer Institute. The purpose of the INSPIRE trial is to see whether avoiding preventative radiation to the groin in patients with normal sentinel node biopsy and PET-CT, is at least as effective in treating cancer as giving preventative radiation to the groin for patients with anal canal cancer. The investigators also want to know if avoiding radiation to the groin will cause fewer side effects and better quality of life.
Cancer Exercise: Evaluation of a Mobile App in Breast Cancer Survivors
Exercise is beneficial for breast cancer survivors in alleviating treatment side effects, improving quality of life, and possibly survival. Exercise guidelines for cancer survivors recommend 150 minutes of moderate-intensity or 75 minutes of vigorous-intensity aerobic exercise per week, and two days of resistance training. However, the number of breast cancer survivors meeting those guidelines declines after treatment because of symptoms such as fatigue, depression, muscle loss, and treatment side effects. The COVID-19 pandemic has increased the challenge of maintaining exercise in breast cancer survivors and drastically transformed exercise intervention delivery. Mobile applications (apps) are promising tools with the potential to overcome barriers to exercise such as cost, accessibility, and travel, which may also reduce social health inequalities. Since 2020, the mobile app named Cancer Exercise has been freely available on Smartphones to cancer survivors but it has not been tested to see if it is effective. The planned study will examine whether a mobile app increases exercise levels in breast cancer survivors after treatments, reduces fatigue, and improves physical functions. This study will be an experimental study with participants (n=240) placed into 1 of 2 groups. One group (n=140) will be asked to use the Cancer Exercise mobile app to increase exercise by at least 60 min./week to 150 min./week over 12 weeks. The other group (n=140) will receive a brochure with the exercise guidelines. Exercise will be measured with an objective device called an accelerometer and by self-report questionnaires before and after the intervention and 6 months later. Fatigue and quality of life will be measured by self-report questionnaires. Objective measures of physical functioning will also be obtained. This research will determine whether a simple mobile app helps increase exercise in breast cancer survivors. If useful, mobile apps to increase exercise may be provided to all breast cancer survivors after treatments to help them recover and possibly even improve survival.
Completed Research Studies
Morielli, A.R., Usmani, N., Boule, N.G., Severin, D., Tankel, K., Joseph, K., Nijjar, T., Fairchild, A., & Courneya, K.S. 2021. Feasibility, safety, and preliminary efficacy of exercise during and after neoadjuvant rectal cancer treatment: The EXERT trial. Clin Colo Cancer, 20(3), 216-226.
Neoadjuvant chemoradiation (NACRT) improves outcomes for rectal cancer patients; however, only 15- 27% achieve a pathologic complete response (pCR). EXERT randomized 36 patients to exercise or usual care, and reported no differences in fitness, grade 3/4 toxicities, or treatment completion; however, the exercise group was 3 times more likely to achieve a pCR (56% vs. 18%; p=0.020). EXERT was the first RCT to suggest that exercising during and after NACRT may improve treatment response, a clinically important outcome associated with possible surgical avoidance and improved survival.
Adams, S.C., DeLorey, D.S, Davenport, M.H., Stickland, M.K., Fairey, A.S, North, S., Szczotka, A., & Courneya, K.S. 2017. Effects of high-intensity aerobic interval training on cardiovascular disease risk in testicular cancer survivors: A phase 2 randomized controlled trial. Cancer, 123(20), 4057-4065.
Testicular cancer survivors (TCS) have an increased risk of cardiovascular disease (CVD). We evaluated the effects of high-intensity interval training (HIIT) on CVD risk factors in 63 TCS. HIIT participants attended 99% of the exercise sessions and achieved 98% of the target exercise intensity. HIIT was superior to usual care for improving VO2peak, CVD risk, arterial thickness, arterial stiffness, post-exercise parasympathetic reactivation, inflammation, and low-density lipoprotein. HIITTS was the first RCT to demonstrate the efficacy of HIIT for managing CVD risk in TCS.
Most Significant Studies
Courneya, K.S., Segal, R.J., Mackey, J.R., Gelmon, K., Reid, R.D., Friedenreich, C.M., Ladha, A.B., Proulx, C., Vallance, J.K., Lane, K., Yasui, Y., & McKenzie, D.C. (2007). Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: A multicenter randomized controlled trial. J Clin Oncol, 25(28), 4396-404.
START was the first RCT to compare aerobic and resistance exercise to usual care in 242 breast cancer patients receiving chemotherapy. We reported that aerobic and resistance exercise improved aerobic fitness, muscular strength, body fat, lean body mass, self-esteem, and chemotherapy completion rate. START was the first trial to show that exercise improved chemotherapy completion rate, a clinically important finding that has since been replicated in an independent trial. START is the most highly cited RCT in exercise oncology.
Courneya, K.S., Sellar, C.M., Stevinson, C., McNeely, M.L., Peddle, C.J., Friedenreich, C.M., Tankel, K., Basi, S., Chua, N., Mazurek, A., & Reiman, T. (2009). Randomized controlled trial of the effects of aerobic exercise on physical functioning and quality of life in lymphoma patients. J Clin Oncol, 27(27), 4605-12.
HELP was the first RCT to examine exercise in 122 lymphoma patients receiving chemotherapy or no treatments. Results showed that exercise improved fitness and quality of life equally in patients on and off chemotherapy. Moreover, 46% of lymphoma patients in the exercise group had a complete response to chemotherapy compared to 31% in the usual care group. These data were the first to suggest that exercise during chemotherapy may improve treatment response. HELP is the most highly cited exercise RCT in lymphoma patients.
Courneya, K.S., Mackey, J.R., Bell, G.J., Jones, L.W., Field, C.J., & Fairey, A.S. (2003). Randomized controlled trial of exercise training in postmenopausal breast cancer survivors: Cardiopulmonary and quality of life outcomes. J Clin Oncol, 21(9), 1660-68.
REHAB was the first RCT to examine the effects of exercise on rehabilitation after the completion of adjuvant therapy in 53 breast cancer survivors. We reported a 98% exercise adherence rate to 15 weeks of supervised aerobic exercise that resulted in significant improvements in physical fitness, quality of life, fatigue, happiness, and self-esteem. REHAB is the second most highly cited RCT in exercise oncology.
Courneya, K.S., McKenzie, D.C., Mackey, J.R., Gelmon, K., Friedenreich, C.M., Yasui, Y., Reid, R.D., Cook, D., Jespersen, D., Proulx, C., Dolan, L.B., Forbes, C.C., Wooding, E., Trinh, L., & Segal, R.J. (2013). Effects of exercise dose and type during breast cancer chemotherapy: Multicenter randomized trial. J Natl Cancer Inst, 105, 1821-32.
CARE was the first RCT to compare different types and doses of exercise in 301 breast cancer patients receiving chemotherapy. CARE showed that the higher dose exercise interventions were safe, did not interfere with chemotherapy completion, and were modestly superior to a standard dose of aerobic exercise for patient-reported physical functioning, bodily pain, endocrine symptoms, muscular strength, and aerobic fitness.
Prostate cancer is the most common cancer in Canadian men. Most prostate cancer patients immediately receive expensive treatments including surgery, radiation therapy, and hormonal therapy that may cause serious side effects such as urinary or sexual dysfunction. To avoid these negative impacts, a clinical practice called “active surveillance” has been introduced for low-risk prostate cancer patients. In active surveillance, men do not receive any treatments unless or until their prostate cancer becomes clinically significant. Unfortunately, about a half of men eventually do need medical treatments due to tumour progression and/or fear of cancer progression.
Interventions designed to slow tumour growth and reduce fear of cancer progression would represent a major advance in the care of these men. Exercise has been shown to delay the progression of prostate tumours in animal models by increasing immune function, however, no studies have been done in men with prostate cancer. Moreover, exercise can help manage some symptoms in prostate cancer patients but no study has examined fear of cancer progression. Right now, prostate cancer patients on active surveillance receive limited advice concerning exercise.
The aims of this study are to provide evidence on the effects of exercise on (1) cardiorespiratory fitness, (2) biochemical progression of prostate cancer and cancer-related biomarkers, and (3) patient-reported outcomes including anxiety, fear of cancer progression, quality of life, and symptoms in prostate cancer patients undergoing active surveillance.
Active surveillance is the preferred way of managing men with low-risk prostate cancer but many men still eventually need treatments. Exercise could be an effective intervention that can help men with prostate cancer remain on active surveillance longer by delaying tumour progression and reducing their fear of cancer progression. Ultimately, exercise may play an important role in improving long-term clinical outcomes and save significant medical costs related to prostate cancer treatment.
Kang, D.W., Fairey, A.S., Boule, N.G., Field, C.J., Wharton, S.A., & Courneya, K.S. 2021. Effects of high-intensity interval training on physical fitness and prostate specific antigen in prostate cancer patients on active surveillance: A randomized controlled trial. JAMA Oncol, 7(10), 1487-1495.
Men with low-risk localized prostate cancer are managed with active surveillance (AS) to avoid treatment effects, however, 30%-50% ultimately require treatments. ERASE was the first RCT to examine the effects of exercise in 52 men with prostate cancer on AS. The exercise group attended 96% of the exercise sessions and significantly improved VO2peak, PSA, and PSA velocity.
Resources
Our laboratory has a number of resources that may be helpful if you are a cancer survivor, or are caring for someone who is. Our focus is on the benefits of physical activity and our resources will help you to incorporate this all-important facet of your treatment into your wellness regimen.
All of these resources have been compiled from the numerous studies we have conducted in the Behavioural Medicine Laboratory.
You may download any of the resources in PDF format. Though these are copyrighted texts they are provided here for you to use as you journey to wellness, or support another in theirs.
Exercise for Health Guidebook. Download Exercise for Health: An Exercise Guide for Breast Cancer Survivors© in PDF format or open the guide (below). This guide book is designed to help breast cancer survivors increase their physical activity.
Exercise for Breast Cancer Survivors (PDF)
Nutrition and Physical Activity Guidelines for Cancer Survivors (PDF) - information on healthy eating, taking supplements during treatments and helpful information about keeping physically active. Information from A Cancer Journal for Clinicians.
Canadian Cancer Society - Physical activity guidelines- guidelines to help you determine how much exercise you need for fitness and well-being
Canadian Physical Activity Guidelines for Adults (PDF) - for adults up to 50 years of age from the Canadian Society for Exercise Physiology
Canadian Physical Activity Guidelines for Older Adults (PDF) - for adults over 50 from the Canadian Society of Exercise Physiology
Wellspring, Edmonton - this resource provides emotional, physical, psychological and spiritual support for those faced with a cancer diagnosis
Physical Therapy at the Cross Cancer Institute in Edmonton. Contact the Cross Cancer Institute or your oncologist for details about using this service at the Clinic
The American College of Sports Medicine has guidelines for cancer survivors.
ACSM's physical activity guidelines for older adults.The American College of Sports Medicine provides an excellent resource to help older adults keep fit through physical activity
The American Cancer Society's Nutrition and Physical Activity guidelines - web resources and a downloadable PDF here and on the site are available for your use
Physical Activity Guidelines for Americans from the U.S. Department of Health and Human Services. These guidelines offer a wealth of physical activity manuals for every stage of life, from childhood to adulthood
Alberta Cancer Exercise (ACE) Program for cancer survivors
Who Works in Our Lab
Dr. Kerry Courneya
Director of the Exercise Oncology Research Laboratory
Faculty of Kinesiology, Sport, and Recreation
College of Health Sciences
Graduate Students + Post-Doctoral Fellows
- Fernanda Arthuso
- Myriam Filion
- Stephanie Ntoukas
Staff
- Ki-Yong An, Research Associate
Research Collaborators
- Dr. John R. Mackey (Oncology, Cross Cancer Institute)
- Dr. Christine M. Friedenreich (Medicine, University of Calgary)
- Dr. Roanne Segal (Oncology Rehabilitation, Ottawa Hospital)
- Dr. Donald McKenzie (Exercise Scientist, University of British Columbia)
- Dr. S. Nicole Culos-Reed (Kinesiology, University of Calgary)
- Dr. Margie McNeely (Physical Therapy, University of Alberta)
- Dr. Laura Q. Rogers (Internal Medicine, SIU School of Medicine, Springfield IL)
- Dr. Ron Plotnikoff (Physical Activity and Population Health Education, University of Newcastle, AU)
- Dr. Anna Hawkes (Cancer Council, Queensland, AU)
- Dr. Trish Livingston (School of Nursing, Deakin University, Queensland AU)
- Dr. Lene Thorsen (Psychosocial Oncology and Rehabilitation, University of Oslo, Norway)
