David Olson

Adjunct Professor
Professor, Department of Obstetrics and Gynecology
PhD, St. Louis University
Laboratory Location: 220 Heritage Medical Research Centre
Email address: dmolson@ualberta.ca
Telephone Number: (780) 492-2765
Awards
CIHR Commercialization Grant
Two CIHR Operating Grants characterizing the diagnostic and therapeutic roles of IL-6 & IL-1 in preterm birth
Research Interests / Academic Activities
Preterm Birth
Before the appearance of clinical signs of labour, parturition starts as a silent inflammatory event that morphs into a self-perpetuating amplification of many inflammatory pathways, including a massive infiltration of leukocytes into the uterine and fetal tissues. We (and others) have characterized the mechanisms leading to term and preterm birth (PTB), which are similar but differ in timing and sometimes cause (Figure 1). Ongoing intrauterine inflammation harms the developing fetal organs and can lead to fetal inflammatory response syndrome (FIRS). Babies born with FIRS can suffer many complications that may be fatal or impair their long-term health.
Diagnostics and Therapeutics for Preterm Birth
Clinicians lack the tools to effectively determine which women will have a preterm delivery. Our lab has designed multiple diagnostic strategies, including our leukocyte migration assay. This patented bioassay requires a simple blood test, and it measures the activation and mobilization of isolated peripheral leukocytes to chemotactic homogenate isolated from the fetal membranes. It has been tested in two pilot studies, and in both, the assay determined if a woman would deliver in the next 5-7 days with a positive predictive value of >90%. We are now characterizing the fetal membrane chemoattractant’s composition to standardize and commercialize this assay.
Existing preterm birth drugs target uterine contraction, a strategy ineffective in delaying delivery timing or improving fetal health outcomes. We propose that therapeutics should instead target the upstream regulators of the underlying inflammation. We are investigating the efficacy of new Canadian-made therapeutics designed by our collaborators at the University of Montreal (Dr. Sylvain Chemtob), allosteric antagonists targeting IL-1b (rytvela) and IL-6 (HSJ633). Rytvela treatment in preterm labour-induced mice eliminates the increased rates of PTB, fetal death, and infiltration of leukocytes into the reproductive tissues and fetal brains.
Prenatal Maternal Stress and Preterm Birth
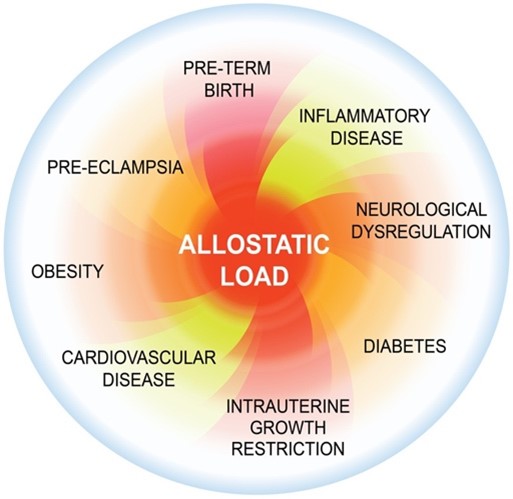
Stress can also influence the timing of delivery. Allostatic load (total accumulated stress) is the physiological and psychological wear and tear that comes from a person’s cumulative experiences of stress over their lifetime (Figure 2). An allostatic load that exceeds a person’s psychological and physiological resilience increases their risk for many disease states, including PTB. We study the physiological and behavioural effects of maternal and paternal prenatal stress on parents and offspring in rats with Prof. Gerlinde Metz (University of Lethbridge). We expose pregnant rats to stressors and measure gestational lengths, maternal and offspring weights, hormone levels, inflammatory factors, brain morphology, metabolic factors, anxiety, and behaviours in these animals and if any changes are passed down epigenetically through multiple generations. We also study women who have experienced stress over a lifetime or through a specific event (e.g., natural disaster) to understand who is at risk for PTB and to develop tools to improve their resilience and achieve better health outcomes.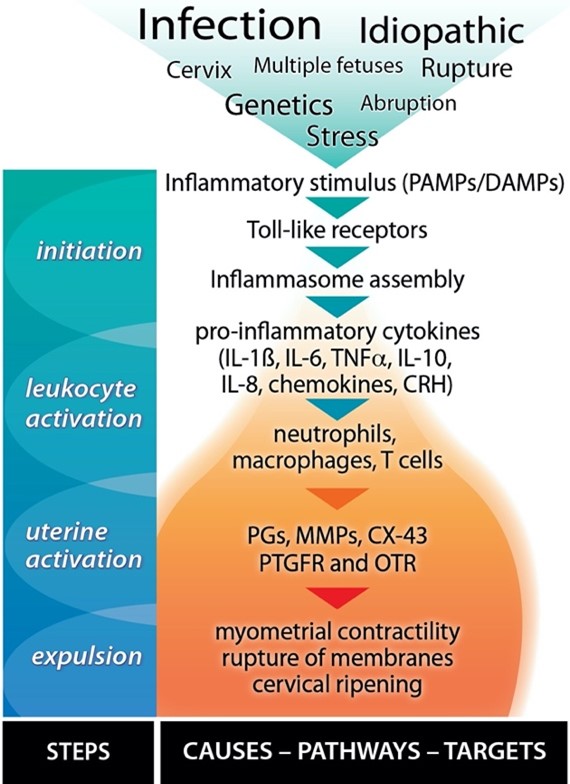
Select Publications
- Habelrih T, Tremblay DÉ, Di Battista E, Hou X, Reuben A, Ferri B, Loiselle SE, Côté F, Abram P, Lubell WD, Leimert KB, Quiniou C, Girard S, Olson DM, Chemtob S. Pharmacodynamic characterization of rytvela, a novel allosteric anti-inflammatory therapeutic, to prevent preterm birth and improve fetal/neonatal outcomes. AJOG. S0002-9378(22)00812-2. (2022) doi: 10.1016/j.ajog.2022.10.007. Epub ahead of print.
- Lopes NA, Falkenberg EA, Wiley C, Patel V, Serrano-Lomelin J, Fang X, Weiler AM, McCreary JK, Metz GAS, Olson DM. Social isolation stress modulates pregnancy outcomes and the inflammatory profile of rat uterus. IJMS. 23(11):6169. (2022) doi: 10.3390/ijms23116169.
- Lee H, Patel V, Onushko M, Fang X, Chemtob S, Olson D. A leukocyte migration assay assists understanding of interleukin-1β-induced leukocyte migration into preterm mouse uterus. Front. Pharmacol. 13:898008. (2022) doi: 10.3389/fphar.2022.898008.
- Leimert KB, Xu W, Princ MM, Chemtob S, Olson DM. Inflammatory amplification: A central tenet of uterine transition for labor. Front. Cell. Infect. Microbiol. 11:660983. (2021) doi: 10.3389/fcimb.2021.660983.
- Verstraeten BSE, Elgbeili G, Hyde A, King S, Olson DM. Maternal mental health after a wildfire: Effects of social support in the Fort McMurray Wood Buffalo Study. Can. J. of Psychiatry. 66(8):710-718. (2021) doi: 10.1177/0706743720970859.
- Coler BS, Shynlova O, Boros-Rausch A, Lye S, McCartney S, Leimert KB, Xu W, Chemtob S, Olson D, Li M, Huebner E, Curtin A, Kachikis A, Savitsky L, Paul JW, Smith R, Adams Waldorf KM. Landscape of preterm birth therapeutics and a path forward. J. Clin. Med. 10(13):2912. (2021) doi: 10.3390/jcm10132912.
- Prairie E, Côté F, Tsakpinoglou M, Mina M, Quiniou C, Leimert K, Olson D, Chemtob S. The determinant role of IL-6 in the establishment of inflammation leading to spontaneous preterm birth. Cytokine Growth Factor Rev. 59:118-130. (2021) doi: 10.1016/j.cytogfr.2020.12.004.
- Olson DM, Metz GAS. Climate change is a major stressor causing poor pregnancy outcomes and child development. F1000 9:F1000 Faculty Rev-1222. (2020) doi: 10.12688/f1000research.27157.1.
- Leimert KB, Verstraeten BSE, Messer A, Nemati R, Blackadar K, Fang X, Robertson SA, Chemtob S, Olson DM. Cooperative effects of sequential PGF2α and IL-1β on IL-6 and COX-2 expression in human myometrial cells. Biology of Reproduction. 100(5):1370-1385. (2019) doi: 10.1093/biolre/ioz029.
- Leimert KB, Messer A, Gray T, Fang X, Chemtob S, Olson DM. Maternal and fetal intrauterine tissue crosstalk promotes proinflammatory amplification and uterine transition. Biol. Reprod. 100(3):783-797. (2019) doi: 10.1093/biolre/ioy232.
- Verstraeten BSE, McCreary JK, Weyers S, Metz GAS, Olson DM. Prenatal two-hit stress affects maternal and offspring pregnancy outcomes and uterine gene expression in rats: match or mismatch? Biol. Reprod. 100(1):195-207. (2019) doi: 10.1093/biolre/ioy166.
- Takeda J, Fang X, Olson DM. Pregnant human peripheral leukocyte migration during several late pregnancy clinical conditions: a cross-sectional observational study. BMC Pregnancy Childbirth. 17(1):16. (2017) doi: 10.1186/s12884-016-1204-5.
Laboratory Members
Senior Scientist:
Kelycia B. Leimert, Ph.D.
Technician:
Carla Sosa Alvarado, M.Sc.
Project Translation Coordinator:
Anna A. Noga, Ph.D.
Post-doctoral Fellow:
Kazumasa Fuwa, M.D., Ph.D.
Graduate Students:
Nayara Antunes Lopes
Tania Rodezno Antunes
Wendy Xu