New study yields significant insight into a major driver of Alzheimer’s Disease
Donna McKinnon - 28 August 2024
In the Dementia Canada: Economic Burden report, issued in 2022 by the Canadian Centre for Economic Analysis, the total annual economic burden of dementia in Canada is estimated to be $40 billion. If the current trend holds, this burden could grow by 275 per cent over the next 30 years. Delaying the onset of dementia by one, five, or 10 years would reduce the annual burden in 2050 by 10, 42, and 70 per cent, respectively.
Under the umbrella of dementia, Alzheimer's Disease (AD) is the most common form of neurodegenerative disease. While environmental factors play a role, approximately 70 per cent of AD risk is attributable to genetics.
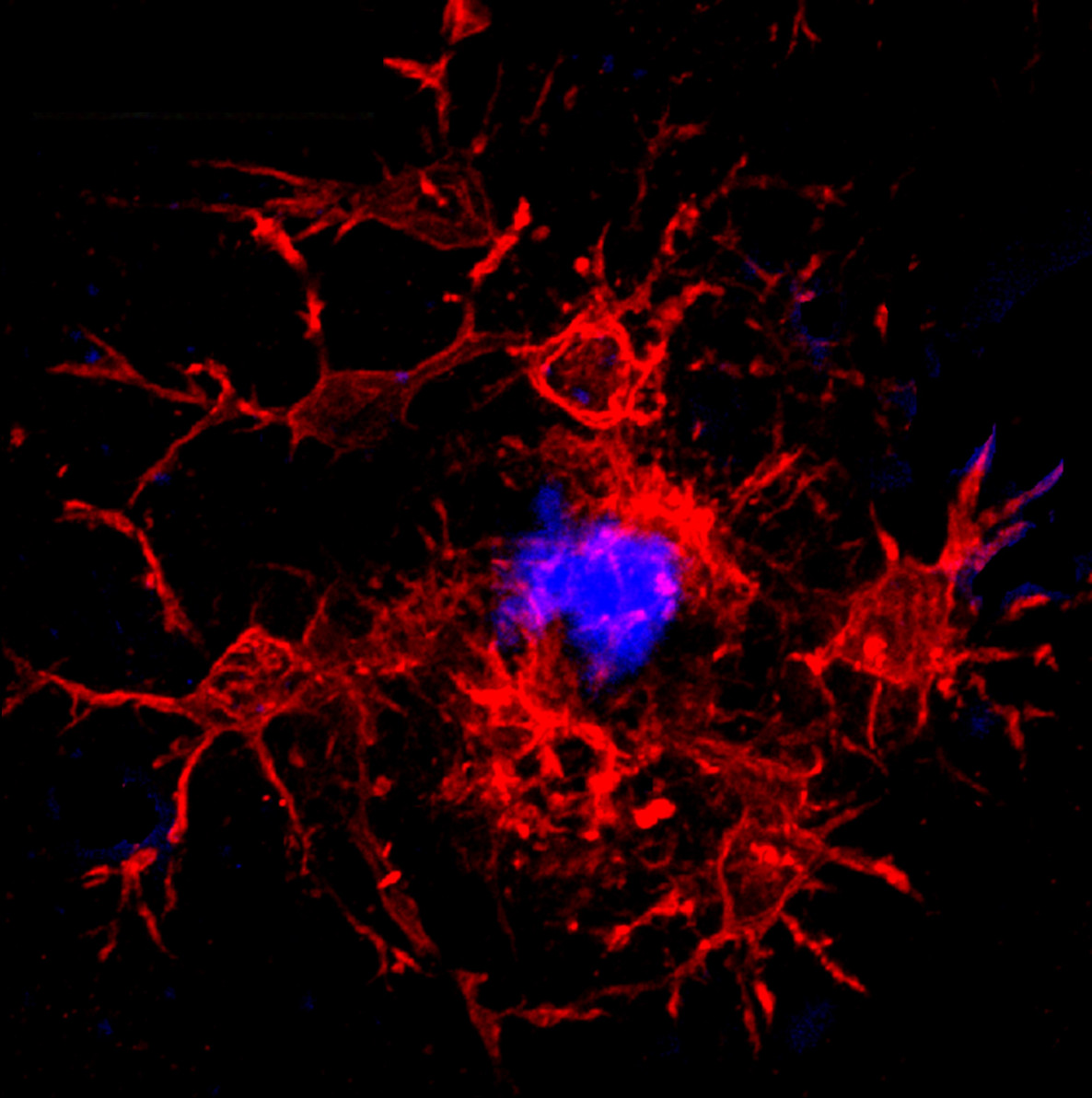
A new study led by Ghazaleh Eskandari-Sedighi, a postdoctoral researcher, and Matthew Macauley in the Department of Chemistry, have shown that CD33, a protein receptor on the surface of microglia (the brain’s immune cells) do not just skew susceptibility to the development of AD in mice, but in its shorter isoform, may also serve as protection against the disease.
Understanding these opposing susceptibility factors is a step toward the development of therapeutic interventions.
The paradox of human CD33
One of the most significant revolutions in the study of complex diseases like Parkinson’s and Alzheimer's, explains Eskandari-Sedighi, is the development of genome-wide association studies (GWAS), which collect and compare millions of complete sets of DNA to find genetic variations and trends associated with particular diseases.
“GWAS hits are like diamonds,” she says. “You dig deep, and when you find something meaningful, you focus on it. CD33 was one of those hits.”
Microglia are crucial for modulating cognition, memory, behaviour, gene expression, and other neurological functions. CD33, an immunomodulatory receptor on the surface of microglia, helps immune cells interact with disease pathogens. Along with APOE e4, it is considered a top-ranked driver in the development of AD, but as their study shows, CD33 in its long isoform (CD33M) and short isoform (CD33m) have dramatic and opposing effects on the development of AD.
The research was, by necessity, cross-disciplinary — spanning the faculties of Science and Medicine & Dentistry with experts in single cell transcriptomics (Jason Plemel), proteomics (Oliver Julien), neurology and behaviour (Qiumin Tan), and biochemistry (Valerie Sim).
“Alzheimer’s is a very complex disease, with many cell types involved,” says Macauley. “It’s impossible to model in a petri dish — and you need to be able to monitor it longitudinally, because realistically, the important part of the development of AD in humans happens 30 years prior to the onset of symptoms. The solution was creating transgenic mice that express either version of CD33 to understand what the microglia are doing in AD pathology, which is when plaques [abnormal clusters of protein fragments] develop and how the microglia is responding.”
As Eskandari-Sedighi notes, in humans both forms of the CD33 protein isoform are present but the ratios differ. When the short isoform dominates, it overrules the negative effect of the long isoform.
A plaque is not just a plaque
“When we started working in this area, we thought of plaques as being bad, but it’s more complicated than that because a lot of people develop plaques who don’t go on to develop AD,” says Macauley. “It’s about how the microglia immune cells respond to those plaques, and how the immune cells then shape the plaques. One of Ghazaleh’s major contributions is that a plaque is not just a plaque — its density is a very important factor. I like to use the garbage analogy — if you let your garbage spread out, you'd be living in garbage. So, we put it in the trash and landfills to keep it contained. That’s what people in the neuroimmunology field postulate the microglia are doing — they are helping to compact the plaques. As Ghazaleh noted, the protective short form of CD33 endowed the microglia with the ability to compact. The converse is also true; the long version of CD33 that makes you more susceptible did the opposite. It made them less compact, and more diffuse.”
Eskandari-Sedighi says Macauley’s novel model of separating the isoforms and studying their impact on microglial cells independent of each other was critical to understanding the mechanism behind one of the most significant drivers of AD.
“Everybody considered CD33 a risk, but our study showed it’s not just risk and absence of risk, it’s actually risk and protection,” she says. “It brings in benefits through the mechanisms that Matt mentioned — making plaques more dense. And that was being missed.”
“A huge percentage of AD risk goes back to immune response and microglia, and that’s why this work, on top of all other research that is currently underway in this field, is important; so that we can understand the mechanisms that lead to disease pathogenesis — basically, whether someone gets the disease or not,” says Eskandari-Sedighi. “If we can understand that, then hopefully we can design therapeutics.”
This study was funded by the Alzheimer Society of Canada and the Alzheimer Society of Alberta and Northwest Territories, in collaboration with Campus Alberta, the Canadian Institutes of Health Research (CIHR) and the Canadian Glycomics Network. Matthew Macauley is a member of the Neuroscience and Mental Health Institute.